News Releases
Konica Minolta Wins the Good Design Award 2025 for the SONIMAGE UX1 and SONIMAGE UX1 TRiFOR Diagnostic Ultrasound Systems and the SONOVISTA LX Transvaginal Diagnostic Ultrasound System
October 15, 2025
Tokyo (October 15, 2025) - Konica Minolta, Inc. (Konica Minolta) today announced that its SONIMAGE UX1 and SONIMAGE UX1 TRiFOR diagnostic ultrasound systems and SONOVISTA LX transvaginal diagnostic ultrasound system won the Good Design Award 2025 of the Japan Institute of Design Promotion (JDP).
* The SONIMAGE UX1, SONIMAGE UX1 TRiFOR, and SONOVISTA LX are available exclusively in Japan.

Evaluation Highlights of the Award-winning Design
Comments from Screening Committee of JDP
These products were highly evaluated for efficiently enabling high-quality ultrasound diagnosis and providing an innovative solution for Point of Care (POC), whose demand has been growing due to staff shortages. The upper limb nerves, which are particularly difficult to identify for novices, can be color-coded and visualized on images thanks to the assistance of proprietary AI and image analysis technologies. This is expected to help standardize the quality of medical care. In ultrasound imaging, it is generally difficult to ensure both a wideband and high sensitivity, but by doing so, these products significantly improve image clarity. The sophisticated design embodies Konica Minolta’s long track record and engineering capabilities.
Features of the Design
A design combining user friendliness, which is essential in POC where bedside testing and treatment are performed, as well as at obstetrics and gynecology settings, and integrity and quality across the series.
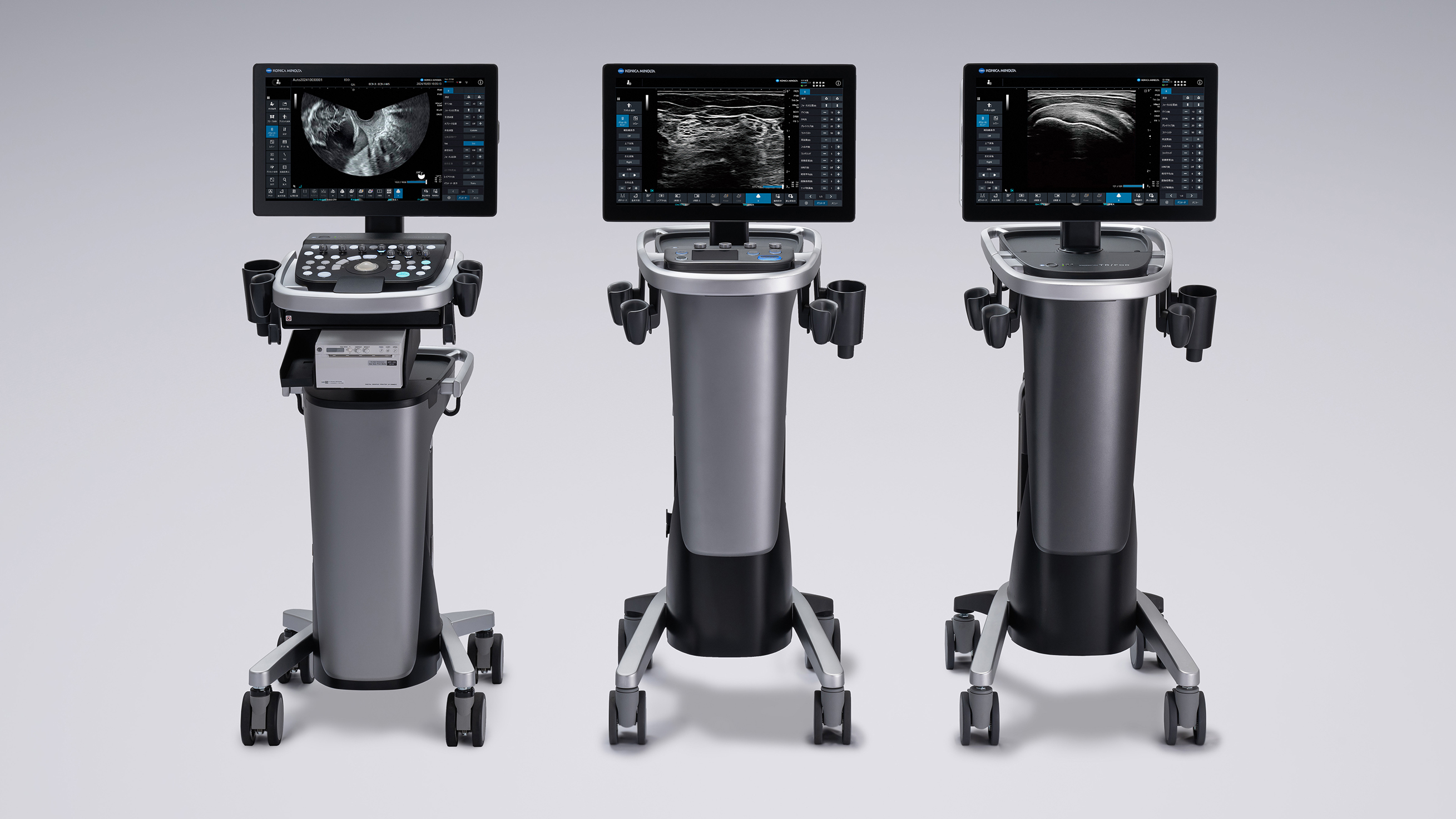
Features of the SONIMAGE UX1 and SONIMAGE UX1 TRiFOR Diagnostic Ultrasound Systems
The SONIMAGE UX1 diagnostic ultrasound system delivers high-definition imaging performance when paired with the X20L linear probe. The SONIMAGE UX1 TRiFOR is an orthopedic model. In addition to providing high-definition imaging, the system offers real-time image sharing to streamline workflows and reduce diagnostic workload.
A range of optional features, including the VisNerve function, which highlights nerves to assist in needle-guided procedures, further enhances diagnostic value.
Features of the SONOVISTA LX Transvaginal Diagnostic Ultrasound System
The SONOVISTA LX transvaginal diagnostic ultrasound system is characterized by an elegant, stylish design, a small footprint, and excellent operability. Dual Sonic Advance, Konica Minolta’s proprietary image enhancement technology, enables highly consistent, high-definition imaging to help improve the accuracy of diagnosis and testing. The transvaginal probe ensures broad coverage with a field of view of 220 degrees, which improves the efficiency of diagnosis and testing and alleviates the burden on patients.
About the Good Design Award
The Good Design Award is the only comprehensive design commendation system in Japan organized by the Japan Institute of Design Promotion. It selects, commends, and publicizes “good designs” in various categories, including products, architecture, software, systems, and services to improve our daily lives and society.
This program has a history spanning approximately more than 60 years since its implementation in 1957 by the Ministry of International Trade and Industry (present-day Ministry of Economy, Trade and Industry) under the name, Good Design Selection System, which was more widely known as the G Mark System.
SONIMAGE, TRIFOR, SONOVISTA, VisNerve, and Dual Sonic are trademarks or registered trademarks of Konica Minolta, Inc. Other product names, service names, and logos are trademarks or registered trademarks of the respective companies.
“SONIMAGE UX1,” “SONIMAGE UX1 TRiFOR,” and “SONOVISTA LX” are the commercial product names of the KUS330 diagnostic ultrasound system (certification No. 306ABBZX00014000).
“X20L” is the commercial product name of the X20L linear probe (certification No. 306ABBZX00013000).
The VisNerve is designed to be used for scanning before treatment and anesthesia using a puncture needle.